Laboratory Information and Management Systems, LIMS, ELN, LES, Document Management
Flexible Laboratory Information and Management Systems for your individual Laboratory Processes

Digitize your lab and optimize your Workflows
Click here for our LIMS solutions:
up2lims
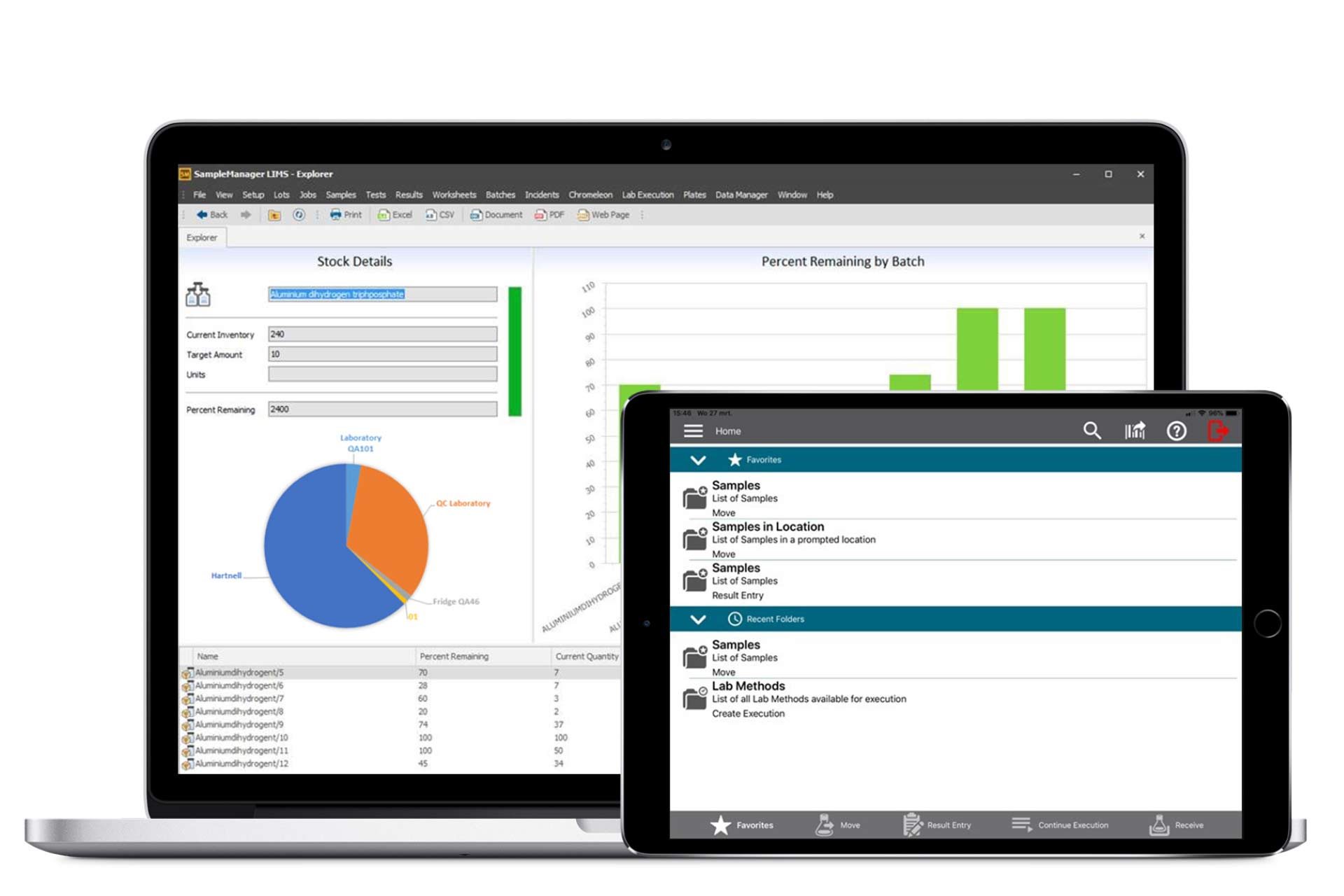
Thermo Scientific™ SampleManager LIMS™
Customers who trust us
How we solve your Challenges

High Time Expenditure
Our Solution
Simplified sample management and processing through system-supported processes.

Unclear Laboratory Processes
Our Solution
Clear operation due to simple system interface.
Variety of different Data Sources
Our Solution
One central system for all laboratory data.

Dependence on IT
Our Solution
Independence through extensive and intuitive configuration options.
High Paper Input
Our Solution
Digitization of the entire processes.
No GMP or ISO compliant LIMS
Our solution
ISO and GMP compliant applicable.
Our LIMS Solutions
With our flexible and process-oriented Laboratory Information Management Systems (LIMS), we enable laboratories to map their individual processes optimally and in compliance with legal and regulatory requirements.
In particular, we offer accredited laboratories as well as laboratories in the GMP environment the possibility to significantly reduce your workload through a gapless and paperless representation of your workflows by means of an extensive basic configuration for special processes and analysis methods.
Your LIMS project in good hands
What distinguishes us

Experience
Over 3 decades experience
from successful implementations.

Quality & Compliance
In-depth knowledge in the regulated environment as well as in the field of stability and bioanalysis.

Future-Proof
Trendsetting solutions for more safety and automation
Flexibility & Personality
Individual service packages tailored to your requirements.

All from a single Source
Complete service portfolio for the entire life cycle of your LIMS.
Team
Competent team of scientists, software engineers and economists.
This is how simple Project Collaboration Works
As a competent partner, we accompany you on your way to digitization.
No two laboratory workflows are alike. The requirements vary greatly from laboratory to laboratory. Pharmaceutical laboratories as well as laboratories in the GMP environment are additionally confronted with high regulatory requirements. The framework conditions for integrating a LIMS into the company’s IT by linking a wide variety of hardware and software systems must also be considered individually in each project.
Together, we analyze your business processes, select the appropriate modules to optimize your laboratory workflow, and ensure that your systems are optimally networked. In this way, we can map exactly what you need in your laboratory to streamline your processes, save time and have legal certainty.
Validation
If validation of the LIMS is required, a validation environment is first created in which the installation of the LIMS, including the configurations performed, takes place under validated conditions.
IQ, OQ and PQ tests are performed in this system. After successful completion of the validation, the system in productive use is installed and a reduced validation is performed.
What our Customers say

Your contact person

Head of Sales – Customer Engagement Manager (CEM)
t + 49 6732 9490-57
e sales@uptodata.com