Knowledge Center
Implementation of ICH M10 Requirements included in StudyReporter Upgrade
Learn how Department of LCMS Bioanalysis at H. Lundbeck A/S has improved reporting performance by upgrading to up to data’s StudyGen 360 Reporter Bioanalytics 3.6 and implementing new regulatory ICH M10 standards within one project.
more »
Bioanalytics
Data Integrity
Reporting
Success Story
Worldwide Clinical Trials selected up to data´s reporting solution “StudyGen 360” to perform Bioanalytical Study Reports
Wörrstadt, Germany – June 15, 2023 – up to data, a long-standing supplier of report generation software for regulatory purposes, announces that it has been selected by Worldwide Clinical Trials, Inc.…
more »
Bioanalytics
Data Integrity
Reporting
Press
Press Release
StudyGen 360 Reporter Bioanalytics SaaS – Bioanalytical Study Data Management without a LIMS
Subscription-based regulatory reporting for pharmaceutical studies, designed with CROs and smaller research labs in mind.
more »
Bioanalytics
Data Integrity
Reporting
Product Flyer
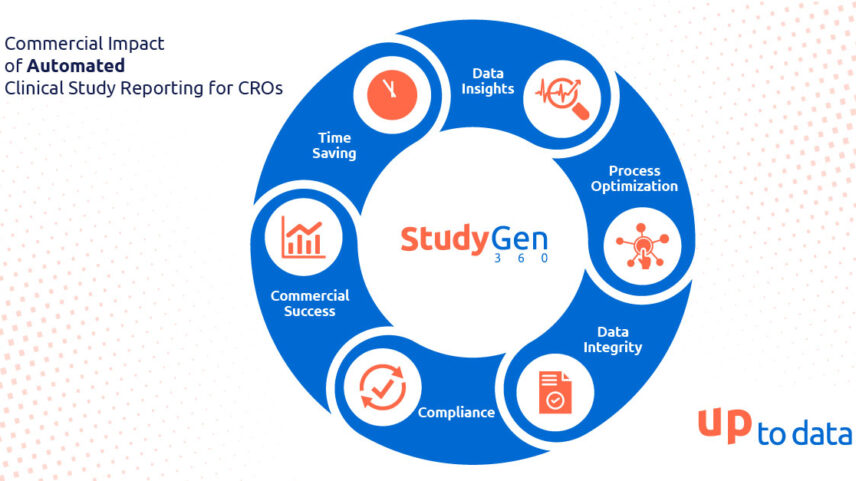
Accelerating Clinical Study Reporting
A Game-Changer for CROs in Enhancing Commercial Success In the landscape of clinical research, the adoption of digitization and automation has emerged as a pivotal force reshaping the way Clinical…
more »
Bioanalytics
Data Integrity
Reporting
Stability
Blog
up to data announces the Release of a cloud-based Bioanalytical Report Writing Solution for CROs
A subscription-based, affordable solution that eliminates upfront implementation costs
generating immediate value.
more »
Bioanalytics
Data Integrity
Reporting
Press Release
Success Story Boehringer Ingelheim
Learn how Boehringer Ingelheim successfully converted their bioanalytical reports to automated reporting in a very short time, and the challenges the world's leading pharmaceutical company faced.
more »
Bioanalytics
Data Integrity
Reporting
Success Story
UCB opted for StudyReporter on Labware LIMS as Stability Reporting Solution
UCB decided to drop the custom development and use StudyReporter, a flagship solution created by up to data. The software is an open and readily configurable off-the-shelf solution that allows for the elaboration of eCTD-conformant stability reports using data from any LIMS or other data source.
more »
Data Integrity
Reporting
Stability
Success Story
End-to-End Management of SAP Stability Data
As one of the world’s leading specialists in immunology and hematology, Biotest AG, a listed biotech company headquartered in Dreieich, Germany, uses SAP QM to document lab data. However, in order to streamline work processes, reduce working time, and minimize data transfer risk, it is essential to implement an end-to-end reporting solution.
more »
Data Integrity
Reporting
Stability
Success Story