GxP-Report Generator based on Lab Devices
StudyGen 360 Reporter Bioanalytics SaaS
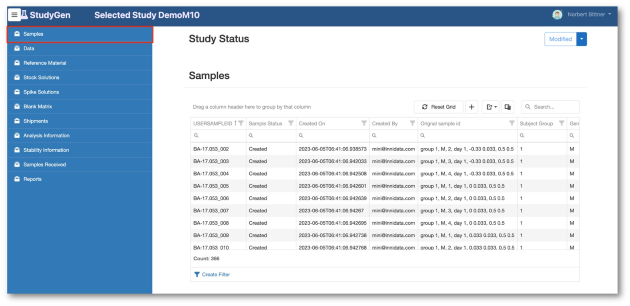
StudyGen 360 provides a centralized approach to study data management where all study-related data, such as sample demographics, instrument data and additional study information, is collected and stored in a central cloud-based data pool.
Study analyse planning, run planning, data review, data analysis are subject to the same central data source, delivering appropiate information for GxP-reporting and non GxP information insights automatically by pushing a button.
Do we need a LIMS when all data is available digitally?
In the digital age, most lab data are already available in a digital format, thus lab data management plays a crucial role in scientific research and analysis. Traditionally LIM-Systems are often used for data management, but they have to be adapted to the existing lab-workflows in a complex way, which leads to high investments for licenses, customizing and hardware that smaller laboratories in particular cannot afford.
By implementing centralized study data management, digital study and device data can be easily collected in a secure, cloud-based data pool, making it available for future data analysis and reporting options, whether for submission purposes or for any type of analysis, such as in a Sponsor-CRO relationship. A LIMS is no longer mandatory.
Increase the efficiency of your study report generation workflows for regulatory approval, accelerate your laboratory processes and subject them to regulatory requirements especially with regards to the new ICH M10 guideline.
StudyReporter Bioanalytics SaaS
Optimization of your Study Reporting Workflow
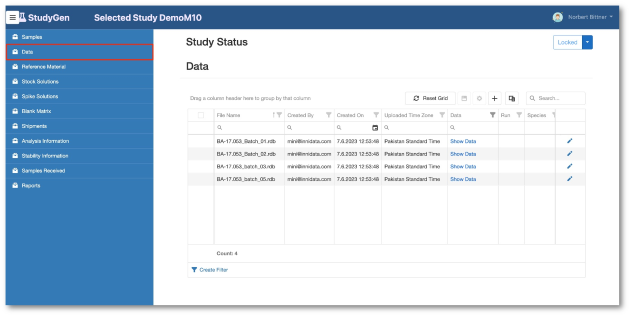
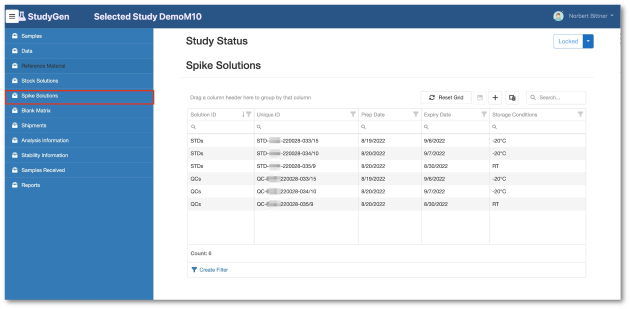
Analytical Report, Tox Report, Validation Report, ADA Report, ADA Validation Report
Selection of Reports and Tables
TOP StudyReporter Bioanalytics SaaS Features
Data Management
- Study documentation incl. Sample Demographics.
- Data import from Sciex Analyst (RDB, WIFF, XML), SoftMax Pro, Excel or customer-specific LIMS.
- Flexible assay configuration.
Data Review
Transparent access to raw data.
Reporting
- Graphical Report Template Builder (Excel-like math & stats.)
- Word Document generation
- Available table templates: tox, clinical, validation templates for small and large molecules, immunogenicity, clinical, validation templates.
Digital Report Transmission (SEND)
Through an export interface, data can be digitally transmitted directly in the CDISC “Standard for Exchange of Nonclinical Data” (SEND) based on created submission reports – secured by eSignatures and audit trails for complete control over your processes and traceability.
Support of multiple Output Formats
- Word/Excel/PDF
Efficiency, Data integrity & Compliance
Your Advantages
Automation
Automated solution with full data integrity independent of specific instruments and/or LIMS software.
Flexibility
Data import from various data sources (Analyst, SoftMax Pro, …, Excel, PDF, CSV, Oracle DB, SQL Server, …).
Compliant
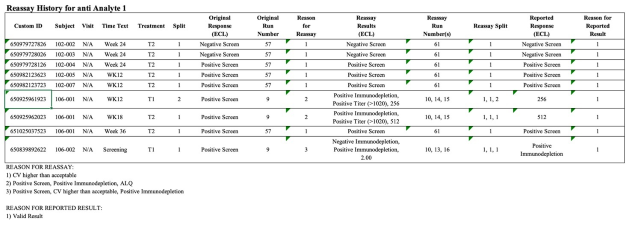
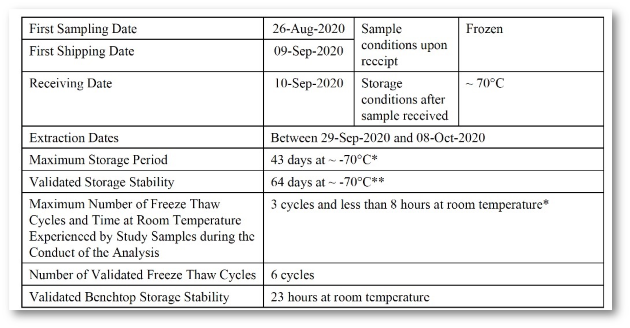
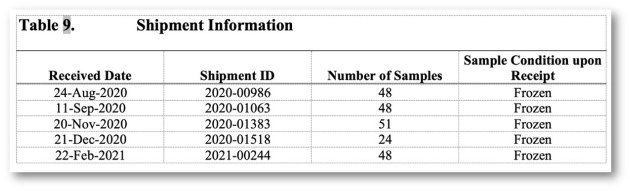
Implementation of all requirements of 21 CFR Part 11, preconfigured reports according to the requirements of ICH M10 and the FDA Guidance (MVA) of 2018, incl. optional reporting of failed runs.

Preconfigured Tables and Graphs
Preconfigured for all bioanalytical analysis types and experiments (toxicology, clinical analyses, validation experiments).

Advanced template editing functions
Integrated layout editor for different templates according to specific sponsor requirements.

Time saving & Transparent
Workflows for optimized support of CRO-Sponsor collaboration (DTA and “Sample Reconciliation”).

Your contact person

Head of Sales – Customer Engagement Manager (CEM)
Phone: + 49 6732 9490-57
Email: sales[at]uptodata.com