The Standard Reporting Solution for Thermo Scientific™ Watson LIMS™
StudyGen 360 Reporter Bioanalytics
As an Watson LIMS Add-On for over twenty years now, iStudyReporter and its successor StudyReporter Bioanalytics is the de-facto gold standard for generating regulatory reports for any type of bioanalytical study from Watson LIMS.
15 of the 25 TOP pharmaceutical companies and leading CROs already successfully use StudyReporter for their report generation.
The Standard Reporting Solution for Watson LIMS™
Whether bioanalytical, clinical or validation studies, for small or large molecules based on LCMS, LBA or immunogenicity measurements. Regulatory bioanalytical documents in line with ICH M10, such as validation and clinical reports can be created easily and quickly, completely eliminating manual steps, manual table formatting, and complex data revalidation checks to ensure data validity.
To meet the specific demands of heterogenous large labs StudyReporter Bioanalytics provides a high degree of interoperability to offer maximum flexibility for data collection and processing from multiple systems (LIMS, ELN, SDMS, MS EXCEL etc.).
Increase the efficiency of your study report generation workflows for regulatory approval, accelerate your laboratory processes and subject them to regulatory requirements especially with regards to the new ICH M10 guideline.
Check out StudyReporter Bioanalytics on Watson LIMS™

Analytical Report, Tox Report, Validation Report, ADA Report, ADA Validation Report
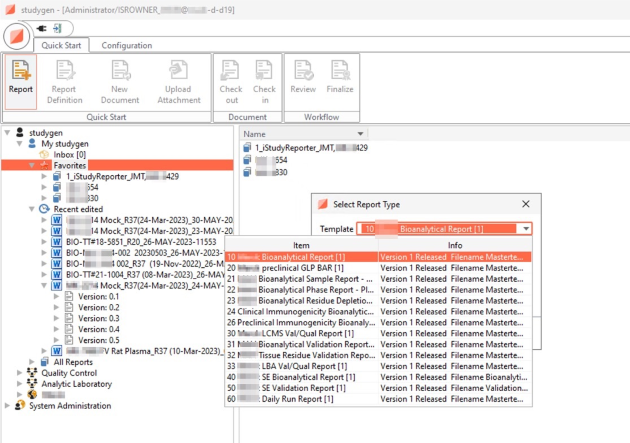
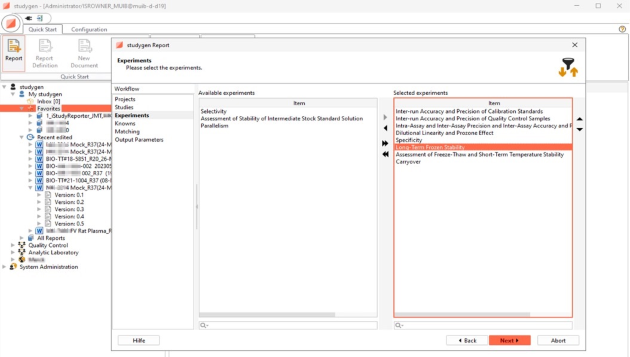
Selection of Reports and Tables
StudyReporter features for Watson LIMS™
Predefined Report Types
Different off-the-shelf tables for specific data preparation and presentation of data from different study types:
Direct Interface
-
Excel
-
Sciex Analyst© Software
-
Thermo Fisher™ Watson LIMS™
Digital Report Transmission (SEND)
Through an export interface, data can be digitally transmitted directly in the CDISC “Standard for Exchange of Nonclinical Data” (SEND) based on created submission reports. Secured by eSignatures and audit trails for complete control over your processes and traceability.
Integrated “Document Life-Cycle" incl. Version Control
-
Full version control
-
All modifications are tracked and audited
-
Transparent document release
-
Configurable document workflows
-
Detailed assignment of rights at group and person level
-
Integrated notification functions
Support of different Output Formats
Ensured Compliance, Data Integrity & Efficiency
Your Advantages
100% Data Integrity
Fully automated solution with direct LIMS integration.
Validated Thermo Scientific™ Watson LIMS™ Interface
Support of all data domains and study types (LCMS, LBA and Immunogenicity).

Implementation of all Requirements of 21 CFR Part 11
Preconfigured reports according to the requirements (as of 2021) of ICH M10 and the FDA Guidance (MVA) of 2018, incl. optional reporting of failed runs.

Preconfigured Tables and Graphs
Preconfigured for all bioanalytical analysis types and experiments (toxicology, clinical analyses, validation experiments).

Configurable Solution with a variety of Templates
LBA/LCMS (tox, clinical, small and large molecule validation template), immunogenicity (clinical validation template).

Time Saving & Transparent
Workflows for optimized support of CRO-Sponsor collaboration (DTA and “Sample Reconciliation”).

Your contact person

Head of Sales – Customer Engagement Manager (CEM)
Phone: + 49 6732 9490-57
Email: sales[at]uptodata.com