Data Integrity in Bioanalytical Sample Handling without a LIMS
StudyGen 360 Lab Sample Management
StudyLab Sample Management is an application that collects and processes data generated in your sample handling process. The focus is on an output-oriented approach for sample management in bioanalytical, study-centered laboratories.
Centralized study data including sample management is available in a cloud-based data platform where all study-related data, such as sample information, assay planning, plate management and instrument data integration, is collected and stored in a central data pool.
Make the most of your clinical study data.
Compliant Lab Data Management without a LIMS
Bioanalytical ICH M10 Lab Workflow at its best
Compliance and complete control of sample management in a bioanalytical study lab is a major challenge especially for smaller CROs or independent labs without LIM- solutions.
Based on the ICH M10 guidelines, the study documentation must include proper handling of the samples, recording of the processing itself and the documentation of the corresponding processing steps – especially in the CRO environment always in accordance with the sponsor’s specifications.
StudyLab Sample Management is specially developed to meet these needs, supporting your lab workflow from the very beginning until the final report generation.
Capturing Data during the Sample Life Cycle
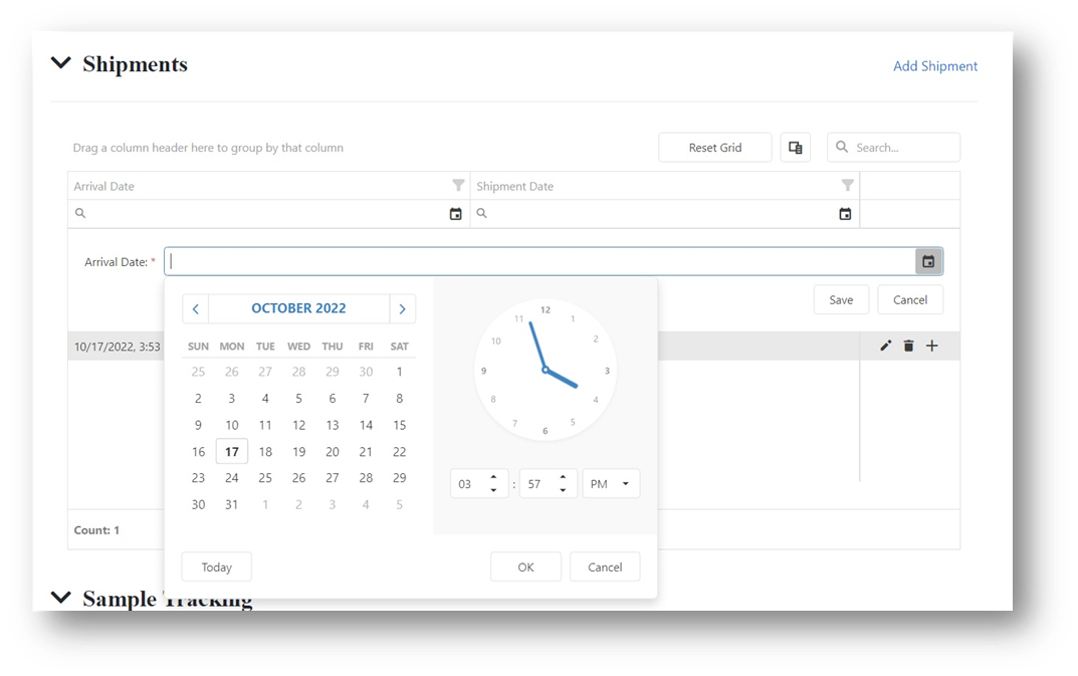
Sample Check-In
- All relevant Sample Information can be easily uploaded into the system.
- Captures all data within the sample receipt process.
- Supports the sample check-in into the system up to the final sample location in your storage system.
Material Management
- Management of stock information about Reference Standard materials and Critical Reagants.
- Collection of information about storage conditions.
- General QC batch information.
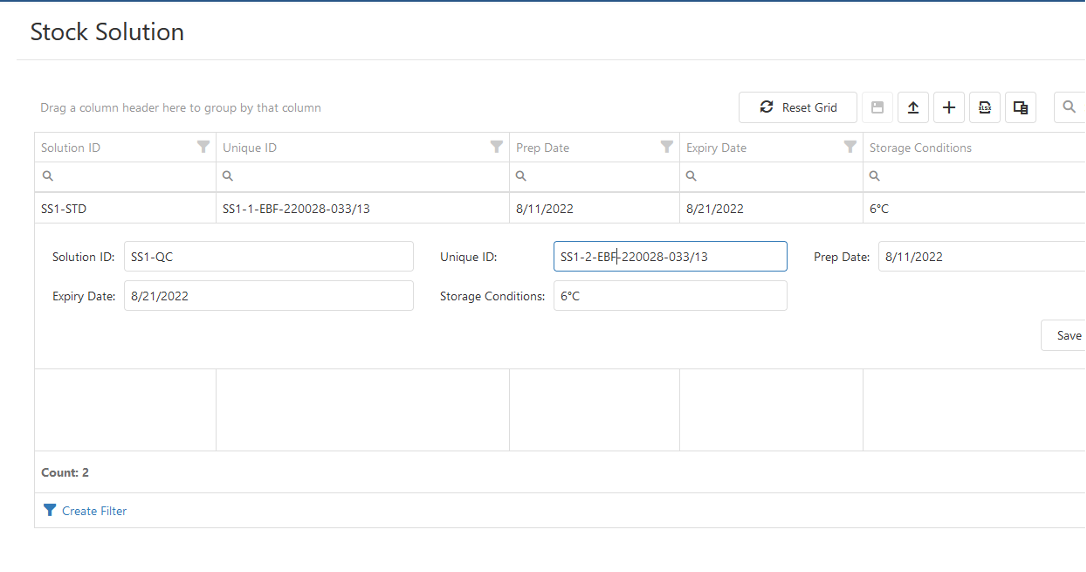
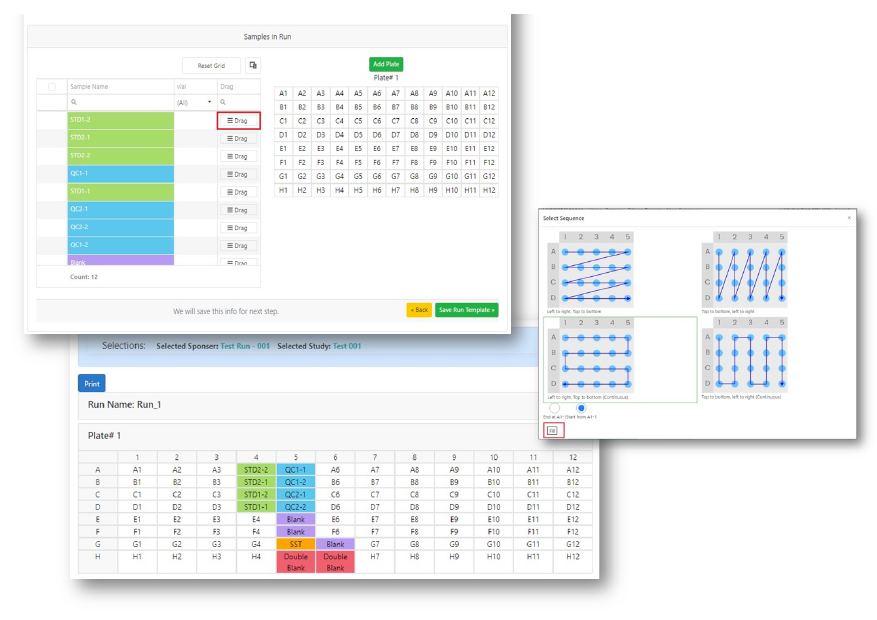
Plate Management
- StudyLab supports you in plate management.
- Plate composition by suggesting how to arrange your samples.
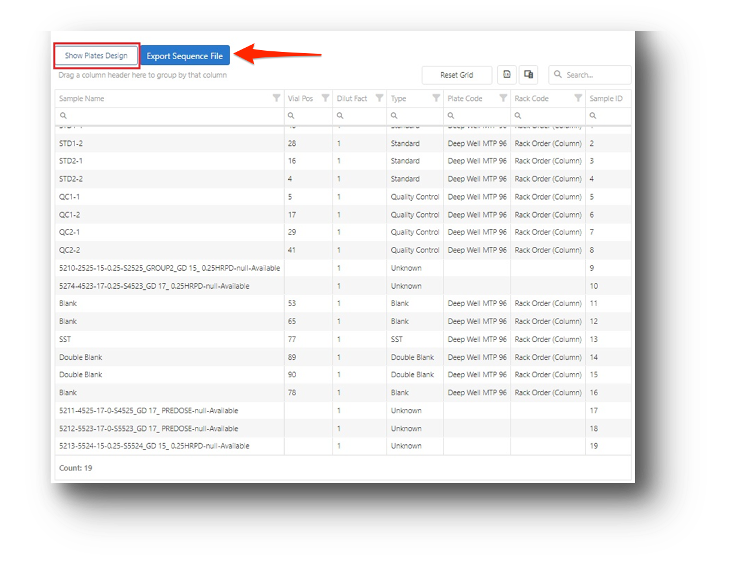
Sequence File and Result Upload
- Create sequence files from plate management.
- Execute runs using device software.
- Upload binary result files.
Your Advantages

Scientific Data in Focus
Strictly follows the science perspective of data. Allows multiple insights in study data. Adaptable to specific data models.
100% End-to-End Data Integrity
Cloud data platform with full data integrit. Independent of specific instruments and/or LIMS software.

Flexibility
Data import from various data sources: Analyst, Shimadzu, SoftMax Pro, …, Excel, PDF, CSV, Oracle DB, SQL Server, …). In-place editor for records
Data Collection of the entire Sample Lifecycle
Creates an extensive study data pool for comprehensive data insights and unique data evaluations.
Compliant and Validated
Comes with validation package. Implementation of all requirements of 21 CFR Part 11, preconfigured reports according to the requirements of ICH M10 and the FDA Guidance (MVA) of 2018

Time saving End-to End solution
From Sample Receipt to the final Study Report. Workflows for optimized support of CRO-Sponsor collaboration incl. DTA and “Sample Reconciliation”.

Your contact person

Head of Sales – Customer Engagement Manager (CEM)
Phone: + 49 6732 9490-57
Email: sales[at]uptodata.com


