knowledgeAccelerating Clinical Study Reporting
 Bioanalytik
Data Integrity
Reporting
Stability
Blog
Bioanalytik
Data Integrity
Reporting
Stability
Blog
A Game-Changer for CROs in Enhancing Commercial Success
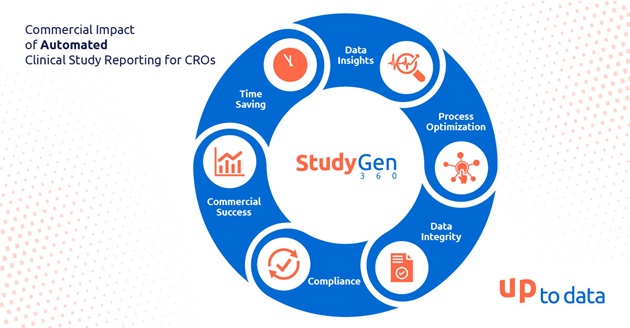
In the landscape of clinical research, the adoption of digitization and automation has emerged as a pivotal force reshaping the way Clinical Research Organizations (CROs) operate. One area where automation is making a sustainable impact is in Clinical Study Reporting. This blog explores the commercial implications of automated lab data Reporting for CROs, focusing on key aspects such as process optimization, data integrity, compliance, time-saving, and data insights examining its influence on the sponsor-CRO relationship.
Process Optimization:
Traditionally, manual processes in Clinical Study Reporting have been time-consuming and prone to errors. Automation streamlines these processes by reducing manual intervention, ensuring consistency, and minimizing the risk of human errors. This optimization not only accelerates the reporting timeline but also enhances the overall efficiency of the CRO’s lab operations. By implementing automated study reporting, CROs can allocate lab resources more strategically, improving their overall workflow and responsiveness.
Data Integrity:
Ensuring data integrity is paramount in clinical research. Automated reporting systems, that integrate into the heterogeneous laboratory data landscape, provide a structured and standardized approach to data management, reducing the likelihood of discrepancies and inaccuracies. By minimizing the chances of data errors, CROs can build a reputation for delivering high-quality and reliable results, consequently attracting more sponsors and establishing long-term partnerships.
Compliance:
Adherence to regulatory requirements is non-negotiable in the clinical research industry. Automated reporting systems are designed to comply with the latest regulatory standards, ensuring that the generated reports meet the necessary guidelines and long-term availability. This not only reduces the risk of regulatory issues but also allows CROs to position themselves as reliable partners for sponsors, instilling confidence in their ability to navigate complex compliance landscapes.
Time Savings:
Time is of the essence in clinical research, and any delays can have cascading effects on project timelines. Automated study reporting drastically reduces the time required for report generation, enabling CROs to meet tight deadlines more effectively. The ability to deliver results promptly enhances a CRO’s competitiveness in the market, attracting sponsors looking for swift and efficient project execution.
Data Insights:
Automated study reporting not only accelerates the reporting process but if it relies furthermore on centralized data management it could also unlock valuable data insights. By leveraging advanced analytics and data visualization tools, CROs can extract meaningful patterns and trends from study data. These insights not only contribute to more informed decision-making but also enable CROs to provide sponsors with a deeper understanding of the study outcomes. This data-driven approach enhances the overall value proposition of CROs, positioning them as partners capable of delivering actionable insights alongside traditional reporting, which has a significant influence on the quality of the clinical study.
Commercial Success in Sponsor-CRO Relationships:
The successful implementation of automated study reporting contributes significantly to the overall commercial success of CROs, especially in their relationships with sponsors. Sponsors seek partners who not only meet regulatory standards but also demonstrate efficiency, accuracy, and a commitment to timelines. By leveraging automation, CROs position themselves as reliable collaborators, fostering stronger relationships with sponsors and increasing the likelihood of repeat business.
Conclusion:
Automated clinical study reporting is a game changer for CROs and offers a multitude of benefits that directly impact their commercial success. From process optimization and improved data integrity to ensuring compliance, saving time and strengthening sponsor-CRO relationships, the benefits of automation are changing the landscape of clinical research. CROs that leverage these technological advances are well positioned to thrive in an increasingly competitive and dynamic environment as the industry’s research needs continue to evolve rapidly towards innovative therapies and drug approaches.
